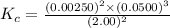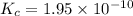Chemistry, 30.03.2020 23:54 dannaasc5475
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions

English, 11.01.2021 20:10



History, 11.01.2021 20:10


Mathematics, 11.01.2021 20:10


World Languages, 11.01.2021 20:10


Physics, 11.01.2021 20:10

Mathematics, 11.01.2021 20:10



Mathematics, 11.01.2021 20:10

Computers and Technology, 11.01.2021 20:10





Mathematics, 11.01.2021 20:10

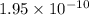
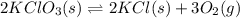
![K_c=\frac{[KCl]^2\times [O_2]^3}{[KClO_3]^2}](/tpl/images/0571/9600/641b5.png)