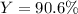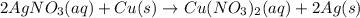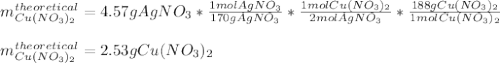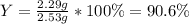For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yields 2.29 gram of copper(II) nitrate What is the percent yield for this reaction?Formula: % yield = (Actual yield/theoretical yield) x 100 2 AgNO3(aq) + Cu(s) à Cu(NO3)2 (aq) + 2 Ag(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yiel...
Questions

English, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00

History, 08.12.2020 22:00



Mathematics, 08.12.2020 22:00


Mathematics, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00





Social Studies, 08.12.2020 22:00


Mathematics, 08.12.2020 22:00

Arts, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00