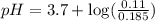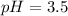Chemistry, 30.03.2020 23:33 erikamaldonado661
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100M sodium formate, HCOONa after the addition of 10.0 mL of 6.00M NaOH to the original buffered solution volume of 500.0 mL.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100...
Questions

Computers and Technology, 10.03.2020 03:30

Computers and Technology, 10.03.2020 03:30


















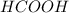 ,
, 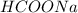 and
and  .
.
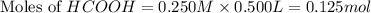
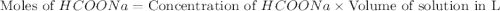
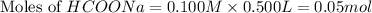
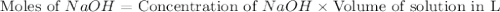
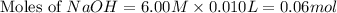
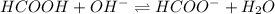
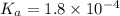
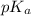 .
.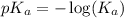
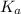 in this expression, we get:
in this expression, we get: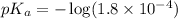
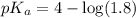
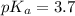
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0571/8655/e961a.png)
![pH=pK_a+\log \frac{[HCOONa]}{[HCOOH]}](/tpl/images/0571/8655/c5edc.png)
![pH=pK_a+\log \frac{[\frac{\text{Moles of HCOONa}}{\text{Volume of solution}}]}{[\frac{\text{Moles of HCOOH}}{\text{Volume of solution}}]}](/tpl/images/0571/8655/fba20.png)