
Chemistry, 30.03.2020 23:33 mathman783
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? 4.10 × 106 s-1 713 s-1 1.36 × 102 s-1 2.65 × 104 s-1 3.69 × 104 s-1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

You know the right answer?
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magni...
Questions



English, 24.07.2019 05:00

Chemistry, 24.07.2019 05:00




Biology, 24.07.2019 05:00


English, 24.07.2019 05:00


History, 24.07.2019 05:00



Biology, 24.07.2019 05:00





Social Studies, 24.07.2019 05:00

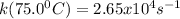
![\frac{k(75.0^0C)}{k(25.0^0C)} =exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]](/tpl/images/0571/8653/57013.png)
![k(75.0^0C)=k(25.0^0C)exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]\\\\k(75.0^0C)=1.35x10^2s^{-1}exp[-\frac{91000J/mol}{8.314J/(mol*K)}(\frac{1}{348.15K}-\frac{1}{298.15K} )]\\\\k(75.0^0C)=2.65 x 10^4 s^{-1}](/tpl/images/0571/8653/debb3.png)


