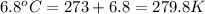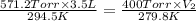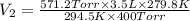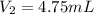Chemistry, 30.03.2020 19:34 madelyngv97
A certain flexible weather balloon contains 3.5 L of helium gas. Initially, the balloon is in WP at 8500ft, where the temperature is 21.5C and the barometric pressure is 571.2 torr. The balloon then is taken to the top of Pike's Peak at an altitude of 14,100ft, where the pressure is 400 torr and the temperature is 6.8C. What is the new volume of the balloon at the top of Pikes Peak?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A certain flexible weather balloon contains 3.5 L of helium gas. Initially, the balloon is in WP at...
Questions

English, 11.03.2021 19:30



Mathematics, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30




Mathematics, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30






Mathematics, 11.03.2021 19:30


Social Studies, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30

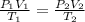
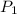 = initial pressure of helium gas , at 8500 feet = 571.2 Torr
= initial pressure of helium gas , at 8500 feet = 571.2 Torr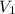 = initial volume of helium gas , at 8500 feet=
= initial volume of helium gas , at 8500 feet= 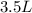
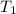 = initial temperature of helium gas , at 8500 feet=
= initial temperature of helium gas , at 8500 feet= 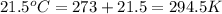
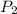 = final pressure of helium gas, at 14,100 feet = 400 Torr
= final pressure of helium gas, at 14,100 feet = 400 Torr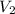 = final volume of helium gas, at 14,100 feet = ?
= final volume of helium gas, at 14,100 feet = ?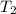 = final temperature of helium gas , at 14,100 feet=
= final temperature of helium gas , at 14,100 feet=