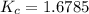Chemistry, 30.03.2020 19:34 jallison61
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially dissociates by the reaction below. 2 NH3(g) equilibrium reaction arrow N2(g) 3 H2(g) At equilibrium, 2.0 mol NH3 remains. What is the value of K for this reaction

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3


Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially di...
Questions

Mathematics, 31.07.2019 22:00

Advanced Placement (AP), 31.07.2019 22:00

Social Studies, 31.07.2019 22:00


History, 31.07.2019 22:00


History, 31.07.2019 22:00

History, 31.07.2019 22:00

World Languages, 31.07.2019 22:00

Business, 31.07.2019 22:00



Social Studies, 31.07.2019 22:00

Biology, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00


Biology, 31.07.2019 22:00

Biology, 31.07.2019 22:00

Social Studies, 31.07.2019 22:00

History, 31.07.2019 22:00

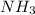 = 4.0 mole
= 4.0 mole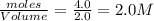
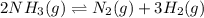
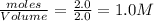
![K_c=\frac{[x]\times [3x]^3}{[(2-2x)]^2}](/tpl/images/0570/9112/70e97.png)
![K_c=\frac{[0.5]\times [3\times 0.5]^3}{[(2-2\times 0.5)]^2}](/tpl/images/0570/9112/97489.png)