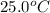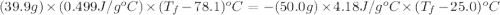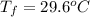A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g⋅°C)? Assume no heat is lost to surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initial...
Questions




















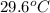
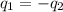
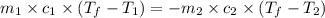
 = specific heat of iron =
= specific heat of iron = 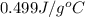
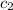 = specific heat of water =
= specific heat of water = 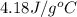
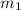 = mass of iron = 39.9 g
= mass of iron = 39.9 g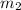 = mass of water =
= mass of water = 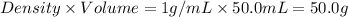
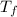 = final temperature of mixture = ?
= final temperature of mixture = ?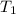 = initial temperature of iron =
= initial temperature of iron = 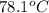
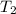 = initial temperature of water =
= initial temperature of water =