

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
For a certain diprotic acid, the pH at one half the volume to the first equivalence point is 4.15 an...
Questions


Mathematics, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30

Biology, 06.07.2019 04:30

English, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30



Mathematics, 06.07.2019 04:30



Physics, 06.07.2019 04:30







Mathematics, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30

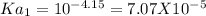 &
& 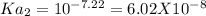
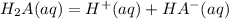 ----------------(i)
----------------(i)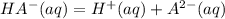 ---------------(ii)
---------------(ii)![Ka_{1}= \frac{[H^{+} ][HA^{-} ]}{[H_{2}A ]}](/tpl/images/0570/7557/d943f.png)
![pH_{1} = -logKa_{1} + log\frac{[Base]}{[Acid]}](/tpl/images/0570/7557/2ee84.png)
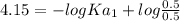
![pH_{2}=-log Ka_{2} + log\frac{[Base]}{[Acid]} \\pH_{2}=-log Ka_{2} + log\frac{[A^{2-} ]}{[HA^{-} ]}\\\\7.22 = - logKa_{2} +log(1)\\\\Ka_{2}=10^{-7.22}= 6.02 X10^{-8}](/tpl/images/0570/7557/9dab2.png)


