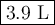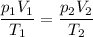A certain flexible weather balloon contains 3.5 L of helium gas. Initially, the balloon is in WP at 8500ft, where the temperature is 21.5C and the barometric pressure is 571.2 torr. The balloon then is taken to the top of Pike’s Peak at an altitude of 14,100ft, where the pressure is 400 torr and the temperature is 6.8C. What is the new volume of the balloon at the top of Pikes Peak?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
A certain flexible weather balloon contains 3.5 L of helium gas. Initially, the balloon is in WP at...
Questions


Mathematics, 24.01.2021 23:20


English, 24.01.2021 23:20

Mathematics, 24.01.2021 23:20



Mathematics, 24.01.2021 23:20


Mathematics, 24.01.2021 23:20










English, 24.01.2021 23:20