
Chemistry, 30.03.2020 04:33 smelcher3900
According to the equation below, how many moles of Ca(OH)2 are required to react with 1.36 mol H3PO4 to produce Ca3(PO4)2? 3Ca(OH)2+2H3PO4⟶Ca3(PO4)2+6H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2


You know the right answer?
According to the equation below, how many moles of Ca(OH)2 are required to react with 1.36 mol H3PO4...
Questions

Mathematics, 03.10.2019 11:00




Computers and Technology, 03.10.2019 11:00


Computers and Technology, 03.10.2019 11:00

Business, 03.10.2019 11:00



Mathematics, 03.10.2019 11:00

Mathematics, 03.10.2019 11:00

Social Studies, 03.10.2019 11:00

Biology, 03.10.2019 11:00




English, 03.10.2019 11:00


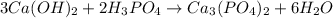
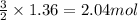 of calcium hydroxide
of calcium hydroxide

