If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen a...

Chemistry, 29.03.2020 01:30 madiiiiiii69
If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen are within phenol. (C6H5OH=94.11g/mol]
0.372 mol H
13.4 mol H.
11.2 mol H
2.23 mol H

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1
You know the right answer?
Questions


Mathematics, 07.06.2020 04:01





Mathematics, 07.06.2020 04:01


Computers and Technology, 07.06.2020 04:01


Mathematics, 07.06.2020 04:01






Mathematics, 07.06.2020 04:01

History, 07.06.2020 04:01


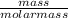
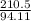 = 2.24mol
= 2.24mol

