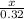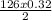Chemistry, 27.03.2020 20:59 kyrabrown33
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M = 78.0 g/mol for Na2O2)
2 Na2O2(s) + 2 H2O(l) → 4 NaOH(aq) + O2(g) ∆Hο = −126 kJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M...
Questions




















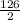 =
=