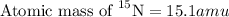The element nitrogen has an atomic weight of 14.0 and consists of two stable isotopes nitrogen-14 and nitrogen-15. The isotope nitrogen-14 has a mass of 14.0 amu and a percent natural abundance of 99.6 %. The isotope nitrogen-15 has a percent natural abundance of 0.370 %. What is the mass of nitrogen-15? amu

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The element nitrogen has an atomic weight of 14.0 and consists of two stable isotopes nitrogen-14 an...
Questions

Computers and Technology, 18.11.2020 09:50

Mathematics, 18.11.2020 14:00

Mathematics, 18.11.2020 14:00


English, 18.11.2020 14:00

English, 18.11.2020 14:00



Social Studies, 18.11.2020 14:00

English, 18.11.2020 14:00

Mathematics, 18.11.2020 14:00

Chemistry, 18.11.2020 14:00




Mathematics, 18.11.2020 14:00

Mathematics, 18.11.2020 14:00

Mathematics, 18.11.2020 14:00


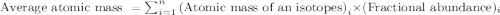 .....(1)
.....(1)
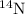 isotope:
isotope: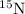 isotope:
isotope:![14.0=[(14.0\times 0.996)+(\text{Atomic mass of }^{15}\textrm{N}\times 0.00370)]](/tpl/images/0566/2813/d6364.png)