Chemistry, 26.03.2020 23:42 nadarius2017
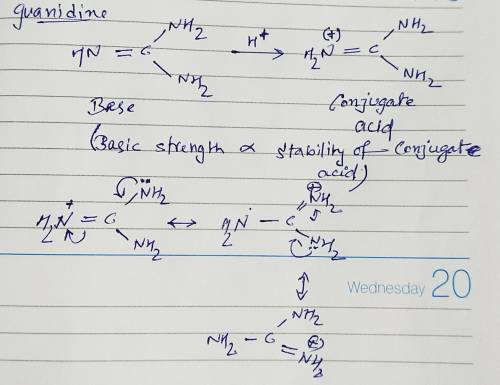
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by drawing the resonance structures of the protonated guanidine. The protonated guanidine (A) has been drawn for you. Draw major resonance structures, one each in boxes B and C, and one minor resonance structure in Box D. Be sure to include the formal charge, lone pairs, and hydrogens on nitrogen for structures B, C, and D.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by draw...
Questions




Mathematics, 22.07.2021 20:10

English, 22.07.2021 20:10

Mathematics, 22.07.2021 20:10



Mathematics, 22.07.2021 20:10

Health, 22.07.2021 20:10

Mathematics, 22.07.2021 20:10

History, 22.07.2021 20:10


Mathematics, 22.07.2021 20:10

Health, 22.07.2021 20:10



Mathematics, 22.07.2021 20:10

Chemistry, 22.07.2021 20:10