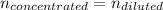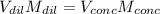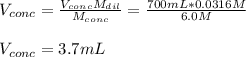Chemistry, 26.03.2020 21:52 MathChic68
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in three steps: Fill a 700.0mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (6.0M) stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in...
Questions



Mathematics, 30.11.2021 02:20



Business, 30.11.2021 02:20



Mathematics, 30.11.2021 02:20





Mathematics, 30.11.2021 02:20



Health, 30.11.2021 02:20

Mathematics, 30.11.2021 02:20


English, 30.11.2021 02:20

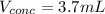
![[H]^+=10^{-pH}=10^{-1.50}=0.0316M](/tpl/images/0565/8791/85748.png)
![[H]^+=[HNO_3]=0.0316M](/tpl/images/0565/8791/de553.png)