
Chemistry, 26.03.2020 22:01 kharmaculpepper
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) 2.22 × 10-2 M/s 1.11 × 10-1 M/s 4.44 × 10-2 M/s 8.88 × 10-2 M/s 5.25 × 10-2 M/s

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the...
Questions


Chemistry, 02.07.2019 10:00

Social Studies, 02.07.2019 10:00

Mathematics, 02.07.2019 10:00

Health, 02.07.2019 10:00


Chemistry, 02.07.2019 10:00

Mathematics, 02.07.2019 10:00

Chemistry, 02.07.2019 10:00

Mathematics, 02.07.2019 10:00

Chemistry, 02.07.2019 10:00

Social Studies, 02.07.2019 10:00

Social Studies, 02.07.2019 10:00

Business, 02.07.2019 10:00

History, 02.07.2019 10:00



History, 02.07.2019 10:00


Mathematics, 02.07.2019 10:00

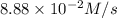
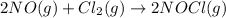
![-\frac{1d[NO]}{2dt}=-\frac{d[Cl_2]}{dt}=+\frac{1d[NOCl]}{2dt}](/tpl/images/0565/9272/04d4d.png)
![\frac{-d[Cl_2]}{dt}]=4.44\times 10^{-2}M/s](/tpl/images/0565/9272/271c7.png)
![-\frac{1d[NO]}{dt}=2\times {\frac{-d[Cl_2]}{dt}=2\times 4.44\times 10^{-2}M/s=8.88\times 10^{-2}M/s](/tpl/images/0565/9272/dea34.png)


