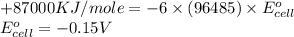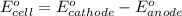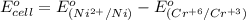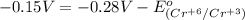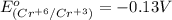3ni2+(aq) + 2 cr(oh)3(s) + 10 oh− (aq) → 3 ni(s) + 2 cro42−(aq) + 8 h2o(l) δg∘ = +87 kj/mol given the standard reduction potential of the half-reaction ni2+(aq) + 2 e− → ni(s) e∘red = -0.28 v, calculate the standard reduction potential of the half-reaction cro42−(aq) + 4 h2o(l) + 3 e− → cr(oh)3(s) + 5 oh−(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
3ni2+(aq) + 2 cr(oh)3(s) + 10 oh− (aq) → 3 ni(s) + 2 cro42−(aq) + 8 h2o(l) δg∘ = +87 kj/mol given th...
Questions

Mathematics, 21.09.2021 22:00


Mathematics, 21.09.2021 22:00


Mathematics, 21.09.2021 22:00


Mathematics, 21.09.2021 22:10


Mathematics, 21.09.2021 22:10


Mathematics, 21.09.2021 22:10



Biology, 21.09.2021 22:10


Mathematics, 21.09.2021 22:10

Mathematics, 21.09.2021 22:10



Mathematics, 21.09.2021 22:10

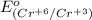 is -0.13 V.
is -0.13 V.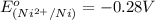
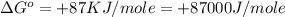 (1 KJ = 1000 J)
(1 KJ = 1000 J)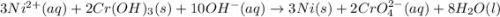
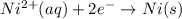
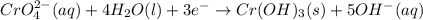
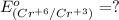
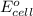 by using formula,
by using formula,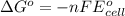
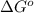 = Gibbs's free energy
= Gibbs's free energy