
Chemistry, 26.03.2020 21:12 torquishag
Consider the following reaction in which all reactants and products are gases. 1.00 mol of A and 2.00 mol of B are placed in a 5.0-liter container. After equilibrium has been established, 0.50 mol of D is present in the container. Calculate the equilibrium constant, Kc, for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
Consider the following reaction in which all reactants and products are gases. 1.00 mol of A and 2.0...
Questions


Biology, 19.02.2022 01:50





Spanish, 19.02.2022 02:00

Business, 19.02.2022 02:00

Computers and Technology, 19.02.2022 02:00


SAT, 19.02.2022 02:00


Chemistry, 19.02.2022 02:00

Chemistry, 19.02.2022 02:00


History, 19.02.2022 02:00

Mathematics, 19.02.2022 02:00



History, 19.02.2022 02:00

![K_{c} = \frac{[D]}{[A][B]}](/tpl/images/0565/8107/95178.png) =
= 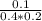 =1.25
=1.25

