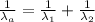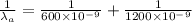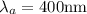Chemistry, 26.03.2020 21:12 ayoismeisjuam
An atom in its ground state is excited when it absorbs a single photon of light. The atom then relaxes back to the ground state by emitting two photons, the first, an orange photon at 600 600 nm, and the second, an infrared photon at 1200 1200 nm. What is the wavelength of the absorbed photon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
An atom in its ground state is excited when it absorbs a single photon of light. The atom then relax...
Questions

Mathematics, 06.11.2019 17:31

Biology, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31

Social Studies, 06.11.2019 17:31

English, 06.11.2019 17:31


Biology, 06.11.2019 17:31

English, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31


English, 06.11.2019 17:31

Computers and Technology, 06.11.2019 17:31




Mathematics, 06.11.2019 17:31




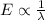 -(1)
-(1)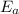 ) is equal to the sum of energies emitted (
) is equal to the sum of energies emitted (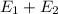 )
)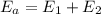
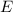 and
and 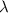 ,
,