Chemistry, 25.03.2020 05:38 codyfore141
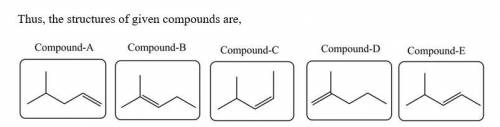
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm–1): Compound A: 912 (s), 994 (s), 1643 (s), 3077 (m) Compound B: 833 (s), 1667 (w), 3050 (weak shoulder on C–H absorption) Compound C: 714 (s), 1665 (w), 3010 (m) Compound D: 885 (s), 1650 (m), 3086 (m) Compound E: 967 (s), no absorption 1600–1700, 3040 (m) The alkene structures are given below. Identify each compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spect...
Questions




Biology, 26.06.2019 01:00

Social Studies, 26.06.2019 01:00




Mathematics, 26.06.2019 01:00

Physics, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00


History, 26.06.2019 01:00

Chemistry, 26.06.2019 01:00

Health, 26.06.2019 01:00



Health, 26.06.2019 01:00


Mathematics, 26.06.2019 01:00