
Chemistry, 25.03.2020 05:05 chonawilson4
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nitrogen dioxide is also produced in the reaction.) What is the enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature (R = 0.0821 L atm/mol K)? [ΔH°f(NO) = 90.4 kJ/mol; ΔH°f(NO2) = 33.85 kJ/mol; ΔH°f(O3) = 142.2 kJ/mol]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nit...
Questions


Mathematics, 13.11.2019 08:31


Mathematics, 13.11.2019 08:31

Mathematics, 13.11.2019 08:31



Mathematics, 13.11.2019 08:31

Biology, 13.11.2019 08:31

Biology, 13.11.2019 08:31







Mathematics, 13.11.2019 08:31

Mathematics, 13.11.2019 08:31

Mathematics, 13.11.2019 08:31

Mathematics, 13.11.2019 08:31


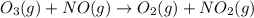
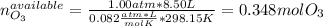

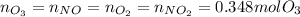
![\Delta _RH=n[\Delta _fH_{products}-\Delta _fH_{reagents}]\\\Delta _RH=0.348mol*[33.85 kJ/mol+0kJ/mol-142.2 kJ/mol-90.4 kJ/mol]\\\Delta _RH=-69.165kJ](/tpl/images/0562/4480/3a1de.png)


