

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
HCl solution, about 250 mL in volume. The pH of the solution is 1.37. Calculate the molar concentrat...
Questions

History, 28.05.2021 09:10



Arts, 28.05.2021 09:10


Mathematics, 28.05.2021 09:10

Mathematics, 28.05.2021 09:10

Mathematics, 28.05.2021 09:10


Arts, 28.05.2021 09:20

Mathematics, 28.05.2021 09:20

Law, 28.05.2021 09:20

Mathematics, 28.05.2021 09:20

Mathematics, 28.05.2021 09:20

English, 28.05.2021 09:20

Medicine, 28.05.2021 09:20

Computers and Technology, 28.05.2021 09:20

Mathematics, 28.05.2021 09:20

Geography, 28.05.2021 09:20

Mathematics, 28.05.2021 09:20

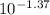 = 0.042 molar
= 0.042 molar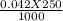 = 0.01 molar
= 0.01 molar

