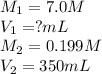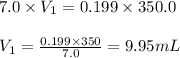Chemistry, 24.03.2020 22:17 sahaitong1844
A chemist must prepare 300.0mL of nitric acid solution with a pH of 0.70 at 25°C. He will do this in three steps: Fill a 300.0mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (7.0M) stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
A chemist must prepare 300.0mL of nitric acid solution with a pH of 0.70 at 25°C. He will do this in...
Questions


History, 27.10.2019 17:43

Chemistry, 27.10.2019 17:43


Mathematics, 27.10.2019 17:43



Mathematics, 27.10.2019 17:43


Mathematics, 27.10.2019 17:43



Mathematics, 27.10.2019 17:43

Arts, 27.10.2019 17:43

World Languages, 27.10.2019 17:43




Health, 27.10.2019 17:43

Chemistry, 27.10.2019 17:43

![pH=-\log[H^+]](/tpl/images/0561/6942/cf945.png)
![0.70=-\log[H^+]](/tpl/images/0561/6942/ee64e.png)
![[H^+]=10^{-0.70}=0.199M](/tpl/images/0561/6942/c6916.png)
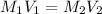
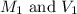 are the molarity and volume of the concentrated nitric acid solution
are the molarity and volume of the concentrated nitric acid solution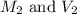 are the molarity and volume of diluted nitric acid solution
are the molarity and volume of diluted nitric acid solution