
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data were obtained: Time (s) [C4H6] (mol/L) 195 1.6 3 1022 604 1.5 3 1022 1246 1.3 3 1022 2180 1.1 3 1022 6210 0.68 3 1022 Assuming that Rate 52 D3C4H64 Dt determine the form of the rate law, the integrated rate law, and the value of the rate constant for this reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data wer...
Questions

English, 16.04.2020 00:37

Mathematics, 16.04.2020 00:37





Social Studies, 16.04.2020 00:37




History, 16.04.2020 00:37


Social Studies, 16.04.2020 00:37

Chemistry, 16.04.2020 00:37

Mathematics, 16.04.2020 00:37

Physics, 16.04.2020 00:37



Mathematics, 16.04.2020 00:37

English, 16.04.2020 00:37

![k[C_4H_6]^2](/tpl/images/0561/6934/d2fdb.png)
![\frac{1}{[C_4H_6]}=\frac{1}{[C_4H_6]_0}+kt](/tpl/images/0561/6934/38768.png)
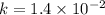
![\frac{1}{[C_4H_6]}](/tpl/images/0561/6934/fd07e.png) and the reaction is second order hence we get the rate law from
and the reaction is second order hence we get the rate law from ![k[A]^n](/tpl/images/0561/6934/3c428.png) .
. ![\frac{1}{[A]}=\frac{1}{[A]_0} +kt](/tpl/images/0561/6934/aad88.png) where A is
where A is 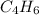 .
. 

