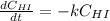Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...

Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys this rate law.
Rate= 8.74 x 10^-4 s^1
Suppose a vessel contains HI at a concentration of 0.330M. Calculate the concentration of HI in the vessel 800 seconds later. You may assume no other reaction is important. Round your answer to significant digit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
Questions


History, 05.09.2019 19:30



Mathematics, 05.09.2019 19:30


Mathematics, 05.09.2019 19:30






Chemistry, 05.09.2019 19:30

Mathematics, 05.09.2019 19:30


Mathematics, 05.09.2019 19:30


Mathematics, 05.09.2019 19:30


Mathematics, 05.09.2019 19:30