
Chemistry, 24.03.2020 19:49 Lovelybunny321
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH3NH3+ + OH- The value of the ionization constant, Kb, is 5.25 x 10 –4. Methylamine reacts to form salts such as methylammonium nitrate, (CH3NH3+)(NO3-). a. Calculate the hydroxide ion concentration, [OH-] of a 0.125 molar aqueous solution of methylamine.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3


Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH...
Questions

Social Studies, 17.10.2021 01:30



Spanish, 17.10.2021 01:30

Chemistry, 17.10.2021 01:30



Mathematics, 17.10.2021 01:30

Biology, 17.10.2021 01:30


Mathematics, 17.10.2021 01:30

Biology, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30


English, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

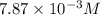
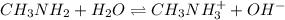
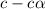
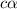
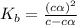
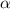 = ?
= ?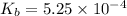
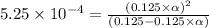
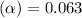
![[OH^-]=c\times \alpha](/tpl/images/0561/1862/0ea5e.png)
![[OH^-]=0.125\times 0.063=7.87\times 10^{-3}M](/tpl/images/0561/1862/08078.png)


