
Chemistry, 24.03.2020 18:13 belladaviau1338
Iron(II) can be oxidized by an acidic K2Cr2O7 solution according to the net ionic equation: Cr2O72− + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O If it takes 35.5 mL of 0.0250 M K2Cr2O7 to titrate 25.0 mL of a solution containing Fe2+, what is the molar concentration of Fe2+ in the original solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

You know the right answer?
Iron(II) can be oxidized by an acidic K2Cr2O7 solution according to the net ionic equation: Cr2O72−...
Questions


Mathematics, 23.10.2020 21:40


Mathematics, 23.10.2020 21:40

Mathematics, 23.10.2020 21:40

Computers and Technology, 23.10.2020 21:40

Social Studies, 23.10.2020 21:40

Mathematics, 23.10.2020 21:40

History, 23.10.2020 21:40

Social Studies, 23.10.2020 21:40

Mathematics, 23.10.2020 21:40





Mathematics, 23.10.2020 21:40


Mathematics, 23.10.2020 21:40


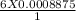 = 0.005325 mole of Fe²
= 0.005325 mole of Fe²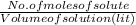
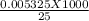 = 1.33 molar
= 1.33 molar

