
Chemistry, 24.03.2020 18:13 doodndns4484
When each of the following equilibria is disturbed by increasing the pressure as a result of decreasing the volume, does the number of moles of reaction products increase, decrease, or remain the same?
1. CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
2. 2CO(g) ⇌ C(s) + CO2(g)
3. N2O4(g) ⇌ 2NO2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
When each of the following equilibria is disturbed by increasing the pressure as a result of decreas...
Questions

Health, 25.02.2021 23:10

History, 25.02.2021 23:10




Mathematics, 25.02.2021 23:10


Mathematics, 25.02.2021 23:10

History, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10




Spanish, 25.02.2021 23:10


Mathematics, 25.02.2021 23:10



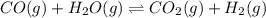 , remain the same.
, remain the same. ,increase.
,increase.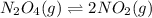 , decrease.
, decrease.


