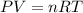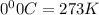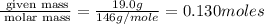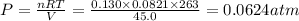Chemistry, 24.03.2020 17:37 connersitte1221l
A reaction between liquid reactants takes place at −10.0°C in a sealed, evacuated vessel with a measured volume of 45.0L. Measurements show that the reaction produced 19.g of sulfur hexafluoride gas. Calculate the pressure of sulfur hexafluoride gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
A reaction between liquid reactants takes place at −10.0°C in a sealed, evacuated vessel with a meas...
Questions



English, 20.11.2020 22:30

English, 20.11.2020 22:30


Mathematics, 20.11.2020 22:30



Mathematics, 20.11.2020 22:30


English, 20.11.2020 22:30


English, 20.11.2020 22:30


Mathematics, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30


English, 20.11.2020 22:30