
Chemistry, 24.03.2020 17:32 bobiscool3698
Nutrasweet is the brand name for the sweetener aspartame. this molecule is the ester of a dipeptide that contains the amino acids phenylalanine and aspartic acid. if aspartame is known to be 27.2% oxygen, by weight, and it has a molecular weight of 292g/mole, how many oxygen atoms must be found in each molecule of aspartame

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Nutrasweet is the brand name for the sweetener aspartame. this molecule is the ester of a dipeptide...
Questions

Mathematics, 19.03.2021 23:30

Mathematics, 19.03.2021 23:30

Mathematics, 19.03.2021 23:30

Mathematics, 19.03.2021 23:30


Mathematics, 19.03.2021 23:30




Mathematics, 19.03.2021 23:30




Mathematics, 19.03.2021 23:30


Mathematics, 19.03.2021 23:30





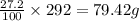
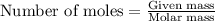
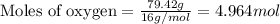
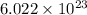 number of atoms
number of atoms number of atoms
number of atoms

