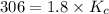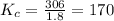Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2


Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction: HbO2(aq) + CO(aq...
Questions





History, 14.07.2020 14:01


Social Studies, 14.07.2020 14:01


History, 14.07.2020 14:01


Mathematics, 14.07.2020 14:01


Mathematics, 14.07.2020 14:01


English, 14.07.2020 14:01





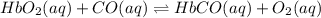
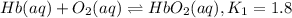
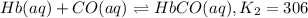
![K_1=\frac{[HbO_2]}{[Hb][O_2]}](/tpl/images/0560/2528/dcc99.png)
![[Hb]=\frac{[HbO_2]}{[K_1][O_2]}](/tpl/images/0560/2528/1a6fb.png) ..[1]
..[1]![K_2=\frac{[HbCO]}{[Hb][CO]}](/tpl/images/0560/2528/abc0a.png) ..[2]
..[2]![K_c=\frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/bc291.png) ..[3]
..[3]![K_2=\frac{[HbCO]}{\frac{[HbO_2]}{[K_1][O_2]}\times [CO]}](/tpl/images/0560/2528/e05b3.png)
![K_2=K_1\times \frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/e36c4.png)
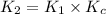 ( using [3])
( using [3])