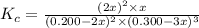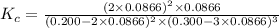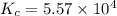In an aqueous solution, iron(III) ions react with iodide ions to give iron(II) ions and triiodide ions, I3-. Suppose the initial concentration of Fe3+ ions is 0.200 M, the initial I- ion concentration is 0.300 M, and the equilibrium concentration of I3- ions is 0.0866 M. What is the value of Kc? 2 Fe3+(aq) + 3 I-(aq) ⇄ 2 Fe2+(aq) + I3-(aq) Kc =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
In an aqueous solution, iron(III) ions react with iodide ions to give iron(II) ions and triiodide io...
Questions

Engineering, 08.12.2019 05:31

Mathematics, 08.12.2019 05:31


English, 08.12.2019 05:31

History, 08.12.2019 05:31



English, 08.12.2019 05:31






Mathematics, 08.12.2019 05:31

Social Studies, 08.12.2019 05:31

Mathematics, 08.12.2019 05:31




English, 08.12.2019 05:31

 is
is 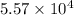 .
.![[Fe^{3+}]=0.200 M](/tpl/images/0559/4796/129ce.png)
![[I^-]=0.300 M](/tpl/images/0559/4796/84de3.png)
![I_3^{-}=[I_3^{-}]=x=0.0866 M](/tpl/images/0559/4796/0d07b.png)
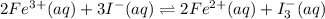
![K_c=\frac{[Fe^{2+}]^2[I_3^{-}]}{[Fe^{3+}]^2[I^-]^3}](/tpl/images/0559/4796/64d23.png)