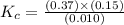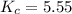Chemistry, 21.03.2020 02:58 alexisfaithsmith
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/l PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1


Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus...
Questions

Computers and Technology, 03.02.2022 14:00


Mathematics, 03.02.2022 14:00



Computers and Technology, 03.02.2022 14:00







Biology, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00


Computers and Technology, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00

Social Studies, 03.02.2022 14:00

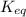 for the reaction is 5.55
for the reaction is 5.55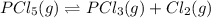
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0557/1142/ffe89.png)