Chemistry, 20.03.2020 12:02 PAADUUUgma
Consider the first-order reaction described by the equation At a certain temperature, the rate constant for this reaction is 5.82 × 10 − 4 s − 1 5.82×10−4 s−1 . Calculate the half-life of cyclopropane at this temperature.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3
You know the right answer?
Consider the first-order reaction described by the equation At a certain temperature, the rate const...
Questions

Health, 26.05.2021 03:10

Mathematics, 26.05.2021 03:10


Mathematics, 26.05.2021 03:10

Mathematics, 26.05.2021 03:10




World Languages, 26.05.2021 03:10



Mathematics, 26.05.2021 03:20

Advanced Placement (AP), 26.05.2021 03:20


French, 26.05.2021 03:20

Chemistry, 26.05.2021 03:20

Health, 26.05.2021 03:20



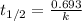
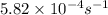
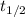 = half life of the reaction = ?
= half life of the reaction = ?