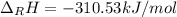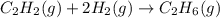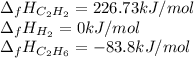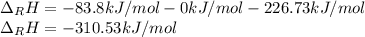Chemistry, 20.03.2020 12:02 tae8002001
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole) the standard enthalpy change ΔH° for the hydrogenation of ethyne (acetylene) to ethane. Just enter a number (no units).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

You know the right answer?
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole)...
Questions

Computers and Technology, 22.02.2021 17:40


English, 22.02.2021 17:40


Mathematics, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40





Business, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40

Biology, 22.02.2021 17:40

Biology, 22.02.2021 17:40

SAT, 22.02.2021 17:40



Spanish, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40