Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
For a first-order reaction, after 2.00 min, 20% of the reactants remain. Calculate the rate constant...
Questions

Advanced Placement (AP), 06.03.2021 01:20

Mathematics, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20



Mathematics, 06.03.2021 01:20

Arts, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20




Mathematics, 06.03.2021 01:20


English, 06.03.2021 01:20

Social Studies, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20

Health, 06.03.2021 01:20

Mathematics, 06.03.2021 01:20

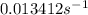
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0556/0145/f1041.png)
![[A_o]](/tpl/images/0556/0145/dc622.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams