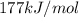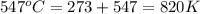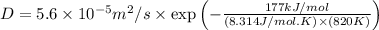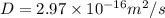Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Calculate the value of the diffusion coefficient D (in m2/s) at 547°C for the diffusion of some spec...
Questions

Mathematics, 15.04.2020 21:36

English, 15.04.2020 21:36

Biology, 15.04.2020 21:36

Mathematics, 15.04.2020 21:36

Mathematics, 15.04.2020 21:36

Mathematics, 15.04.2020 21:36


Mathematics, 15.04.2020 21:36

Biology, 15.04.2020 21:36





Mathematics, 15.04.2020 21:36




Advanced Placement (AP), 15.04.2020 21:36


Business, 15.04.2020 21:36

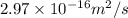
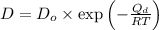
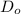 =
= 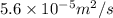
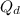 =
=