
Chemistry, 20.03.2020 09:57 nommies005
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl. Write out the dominant equilibrium (including phase labels) that would exist in this "neutralized" solution. HINT: first look at what ions would exist in solution after the non-equilibrium reaction with the strong acid is over. Second, decide if either of these ions is an acid or a base. Lastly, write the equation for this ion acting as an acid or base in water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl....
Questions




English, 24.06.2019 15:00



Spanish, 24.06.2019 15:00

History, 24.06.2019 15:00



Mathematics, 24.06.2019 15:00






Arts, 24.06.2019 15:00



Biology, 24.06.2019 15:00

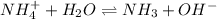
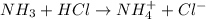
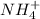 and
and 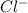 ions exist.
ions exist.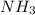 is an weak base and HCl is a strong acid.
is an weak base and HCl is a strong acid.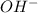 .
.

