
Chemistry, 20.03.2020 01:32 samymaria1344
At a certain temperature, 0.780 mol SO 3 0.780 mol SO3 is placed in a 4.00 L 4.00 L container. 2 SO 3 ( g ) − ⇀ ↽ − 2 SO 2 ( g ) + O 2 ( g ) 2SO3(g)↽−−⇀2SO2(g)+O2(g) At equilibrium, 0.100 mol O 2 0.100 mol O2 is present. Calculate K c .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
At a certain temperature, 0.780 mol SO 3 0.780 mol SO3 is placed in a 4.00 L 4.00 L container. 2 SO...
Questions



Chemistry, 15.04.2020 02:33




Mathematics, 15.04.2020 02:33


Mathematics, 15.04.2020 02:33







English, 15.04.2020 02:34




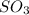 = 0.780 mole
= 0.780 mole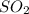 =
= 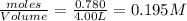
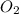 =
= 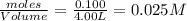
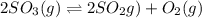
![K_c=\frac{[SO_2]^2\times [O_2]}{[SO_3]^2}](/tpl/images/0555/1079/d826c.png)
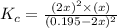
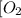 = x = 0.025 M
= x = 0.025 M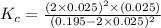
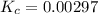
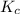 is 0.00297
is 0.00297 


