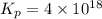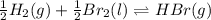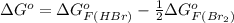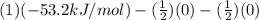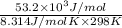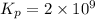Chemistry, 15.04.2020 02:33 karamalqussiri478
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figures. Calculate ΔG o and KP for the following processes at 25°C: (a) H2(g) + Br2(l) ⇌ 2HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.) (b) 1 2 H2(g) + 1 2 Br2(l) ⇌ HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figu...
Questions

Biology, 03.07.2019 22:30


Spanish, 03.07.2019 22:30


History, 03.07.2019 22:30


History, 03.07.2019 22:30


History, 03.07.2019 22:30


History, 03.07.2019 22:30

Arts, 03.07.2019 22:30




Mathematics, 03.07.2019 22:30



Spanish, 03.07.2019 22:30

History, 03.07.2019 22:30

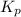 is
is 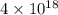 and value of
and value of 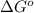 is -106.4 kJ/mol.
is -106.4 kJ/mol.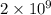 and value of
and value of 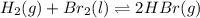
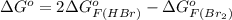
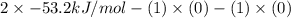
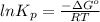
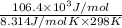
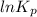 = 42.9
= 42.9