

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

You know the right answer?
Consider a solution formed by mixing 50.0 mL of 0.100 M H2SO4, 30.0 mL of 0.1133 M HOCl, 25.0 mL of...
Questions

Mathematics, 13.09.2020 08:01

Mathematics, 13.09.2020 08:01

Mathematics, 13.09.2020 08:01

Mathematics, 13.09.2020 08:01

French, 13.09.2020 08:01

Mathematics, 13.09.2020 08:01

Arts, 13.09.2020 08:01

Mathematics, 13.09.2020 08:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Biology, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

History, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Biology, 13.09.2020 09:01

History, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

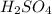
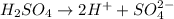
![[H^+]=2\times [H_2SO_4]=2\times 0.100 M= 0.200 M](/tpl/images/0552/5839/cc78c.png)
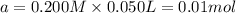
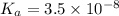
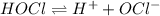
![K_a=\frac{[H^+][OCl^-]}{[HOCl]}](/tpl/images/0552/5839/885cb.png)
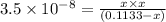
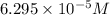
![[H^+]=6.295\times 10^{-5} M](/tpl/images/0552/5839/15b97.png)
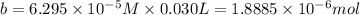
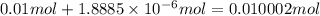
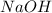
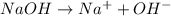
![[OH^-]=1\times [NaOH]=1\times 0.200 M= 0.200 M](/tpl/images/0552/5839/f87f8.png)
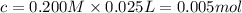
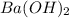
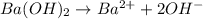
![[OH^-]=2\times [Ba(OH)_2]=1\times 0.200 M= 0.200 M](/tpl/images/0552/5839/8a5dc.png)
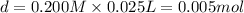
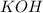
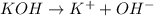
![[OH^-]=1\times [KOH]=1\times 0.170 M= 0.170 M](/tpl/images/0552/5839/40fd0.png)
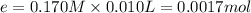
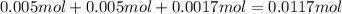
![[OH^-]=\frac{0.001698 mol}{0.050 L+0.030 L+0.025 L+0.025 L+0.010 L}=0.01213 M](/tpl/images/0552/5839/a168c.png)
![pOH=-\log[OH^-]=-\log[0.01213 M]=1.9](/tpl/images/0552/5839/9fef7.png)



