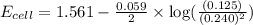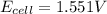Chemistry, 18.03.2020 21:41 cheerthi16
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V and -0.762 V, respectively. Calculate the potential for the following electrochemical cell: Zn(s)|Zn2+(0.125 M)||Ag+(0.240 M)|Ag(s)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2


Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V...
Questions



History, 23.08.2019 04:00



English, 23.08.2019 04:00


Arts, 23.08.2019 04:00

History, 23.08.2019 04:00






Health, 23.08.2019 04:00




Mathematics, 23.08.2019 04:00

Geography, 23.08.2019 04:00

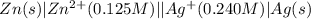
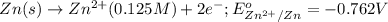
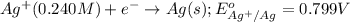 ( × 2)
( × 2)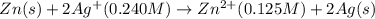
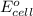 of the reaction, we use the equation:
of the reaction, we use the equation: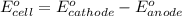
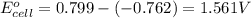
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]}{[Ag^{+}]^2}](/tpl/images/0552/5842/bffc2.png)
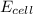 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[Zn^{2+}]=0.125M](/tpl/images/0552/5842/c12f1.png)
![[Ag^{+}]=0.240M](/tpl/images/0552/5842/c82de.png)