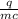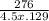Chemistry, 17.03.2020 20:02 fansofboys
A 4.50 g nugget of pure gold absorbed 276 J of heat. the initial temperature was 25.0 degrees C. what was the final temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
A 4.50 g nugget of pure gold absorbed 276 J of heat. the initial temperature was 25.0 degrees C. wha...
Questions

Geography, 04.05.2021 18:10

Mathematics, 04.05.2021 18:10


History, 04.05.2021 18:10

Mathematics, 04.05.2021 18:10


Mathematics, 04.05.2021 18:10

Biology, 04.05.2021 18:10



Mathematics, 04.05.2021 18:10



Mathematics, 04.05.2021 18:10



Mathematics, 04.05.2021 18:10

History, 04.05.2021 18:10

Health, 04.05.2021 18:10

Mathematics, 04.05.2021 18:10