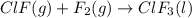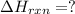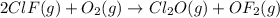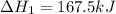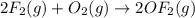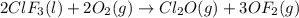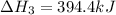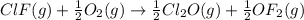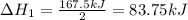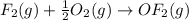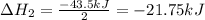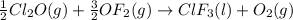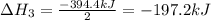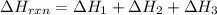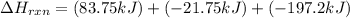Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an important fluorinating agent. Use the following thermochemical equations to calculate ΔH o rxn for the production of ClF3: (1) 2 ClF(g) + O2(g) → Cl2O(g) + OF2(g) ΔHo = 167.5 kJ (2) 2 F2(g) + O2(g) → 2 OF2(g) ΔHo = −43.5 kJ (3) 2 ClF3(l) + 2 O2(g) → Cl2O(g) + 3 OF2(g) ΔHo = 394.1 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
You know the right answer?
Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an importa...
Questions



Computers and Technology, 06.04.2021 01:00






Computers and Technology, 06.04.2021 01:00

English, 06.04.2021 01:00



Computers and Technology, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00




Mathematics, 06.04.2021 01:00

English, 06.04.2021 01:00

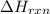 for the reaction is, -135.2 kJ
for the reaction is, -135.2 kJ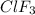 will be,
will be,