
Calculate the pH at the halfway point and at the equivalence point for each of the following titrations. 100.0 mL of 0.60 M titrated by 0.10 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.20 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.15 M pH at the halfway point = pH at the equivalence point ='

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
Calculate the pH at the halfway point and at the equivalence point for each of the following titrati...
Questions

English, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00



English, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00


Mathematics, 03.07.2019 20:00





Health, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00


Biology, 03.07.2019 20:00


Chemistry, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00

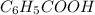 (
(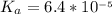 ) titrated by 0.10 M
) titrated by 0.10 M 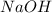
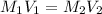 .
.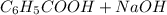 →
→ 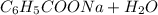
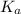 + log
+ log 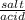
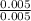 = 4.20
= 4.20

