
Chemistry, 17.03.2020 00:54 kprincess16r
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The lattice constant (a, the edge of the unite cell) has been measured by X-ray crystallography to be 360. pm. Calculate the radius of an atom of M. Be sure your answer has 3 significant digits, and be sure it has the correct unit symbol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3


Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The...
Questions


English, 11.02.2022 21:20

English, 11.02.2022 21:20

History, 11.02.2022 21:20


History, 11.02.2022 21:20

Biology, 11.02.2022 21:20

Chemistry, 11.02.2022 21:20



Mathematics, 11.02.2022 21:20

Mathematics, 11.02.2022 21:20

Mathematics, 11.02.2022 21:20




English, 11.02.2022 21:20


Mathematics, 11.02.2022 21:20

Geography, 11.02.2022 21:20

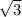 /4
/4

