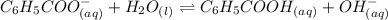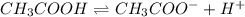Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definitions for acids and bases.
1) An Arrhenius acid is a substance that dissolves in water to produce H+ or H3O+.
2) A Br∅nsted-Lowry base is a proton acceptor.
3) CH3COOH is an Arrhenius base.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definition...
Questions

Biology, 13.10.2020 23:01


Mathematics, 13.10.2020 23:01

History, 13.10.2020 23:01

Arts, 13.10.2020 23:01



Mathematics, 13.10.2020 23:01



History, 13.10.2020 23:01

Mathematics, 13.10.2020 23:01

History, 13.10.2020 23:01





Mathematics, 13.10.2020 23:01


 .
.