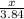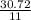Chemistry, 15.03.2020 02:28 poweradampower
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an impurity in
coal, and so burning this fuel in a power plant produces sulfur dioxide (SO), a major air pollutant.
Calculate the mass of sulfur dioxide (SO2) produced when 3.84 mol O2 is reacted with Fes, according to the
equation
4 FeSacs + 11 O.
- 2 Fe2O3(-) + S SO2(e)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an i...
Questions

History, 26.05.2020 23:59

History, 26.05.2020 23:59







Mathematics, 26.05.2020 23:59




History, 26.05.2020 23:59

Mathematics, 26.05.2020 23:59






 =
=