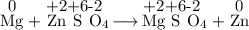Chemistry, 15.03.2020 02:21 jerseygirl6167
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statements regarding this reaction is correct?
The sulfate ion is reduced.
Zinc is the reducing agent.
Magnesium is neither oxidized nor reduced.
Zinc gains two electrons.
Magnesium is the oxidizing agent.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statem...
Questions



Computers and Technology, 18.07.2020 18:01



Spanish, 18.07.2020 18:01







English, 18.07.2020 18:01




English, 18.07.2020 18:01


Mathematics, 18.07.2020 18:01