
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2(g)→2Al2O3(s)
What volume of O2 gas, measured at 787 mmHg and 21 ∘C, is required to completely react with 55.0 g of Al?
Express the volume in liters to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2...
4Al(s)+3O2...
Questions







Computers and Technology, 13.08.2019 02:20













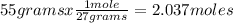
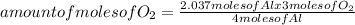
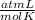 T= 21 C=294 K
T= 21 C=294 K

